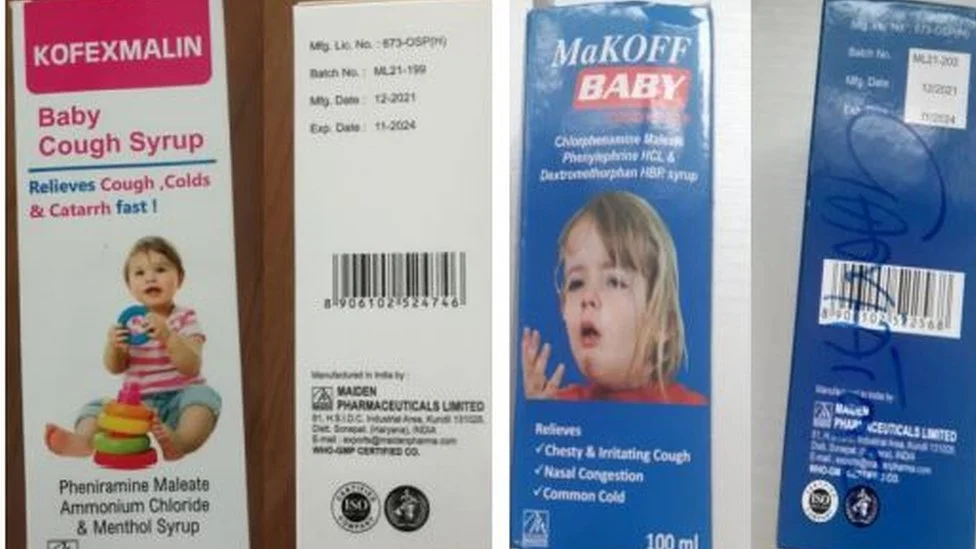
The World Health Organization (WHO) has raised serious concerns about weaknesses in India’s pharmaceutical regulations.
This comes after reports of at least 20 children dying from tainted cough syrups. The incident highlights urgent needs for stronger oversight.
Tragic Impact on Children
In 2025, contaminated cough syrups led to the heartbreaking deaths of 20 young lives in India. Families are left grieving, and communities are demanding answers.
The WHO’s statement underscores the gravity of this public health failure.
Regulatory Shortcomings Exposed
WHO officials pointed to gaps in how India monitors drug quality. Thus, unsafe products reach consumers, endangering vulnerable groups like children. Better testing and enforcement are essential to prevent future tragedies.
Call for Immediate Action
The organization urges Indian authorities to act swiftly. Therefore, reforms should include rigorous inspections and stricter standards. This would protect families and rebuild trust in the healthcare system.
Broader Public Health Implications
Beyond this incident, the issue reveals systemic challenges. Furthermore, weak regulations can affect global supply chains. Strengthening India’s framework benefits everyone, ensuring safer medicines worldwide.
Lessons for Global Health
The WHO’s intervention serves as a reminder for all nations. However, proactive measures can avert similar crises. Investing in drug safety saves lives and fosters confidence in health products.
Path to Safer Medicines
India has the opportunity to lead in reform. By addressing these gaps, the country can enhance its pharmaceutical sector. Ultimately, robust regulations safeguard the most innocent among us.